Functional modulation of developmental signals by a kinesin molecular motor
— KIF16B/Rab14-mediated transport of FGF receptors essential for development of a life
Fibroblast growth factors (FGFs) are secreted proteins which bind to cell surface receptors to evoke proliferation and differentiation signals. It has long been elusive how newly synthesized FGF receptors are presented to the cell surface, but Ueno et al. from Hirokawa lab have now proposed the molecular mechanism as well as physiological relevance through molecular genetics of a microtubule motor, KIF16B. Vesicles containing FGF receptors (FGFRs) are transported from the Golgi apparatus in the cell center toward hub-like recycling endosomes at the vicinity of the cell surface. Activation of Rab14 G-protein on the vesicle facilitates this transport by making it directly bind to the KIF16B molecular motor, which then transports the vesicles along the microtubule track. In early development of embryos, FGF signaling is involved in differentiation of inner cell mass (ICM), which is a primordium of all cells in the fetus. Strikingly, embryos lacking KIF16B or Rab14 activity could not make ICM differentiation or develop the fetus at all, because FGFR2 transport was reduced so that FGF signaling was impaired. Accordingly, biosynthetic intracellular receptor transport through KIF16B and Rab14 has been turned out to be essential for development of a life, and may also generally be involved in modulation of signal transduction for diseases and neuronal functions.
Program member
Nobutaka Hirokawa (Department of Cell Biology and Anatomy, Graduate School of Medicine)
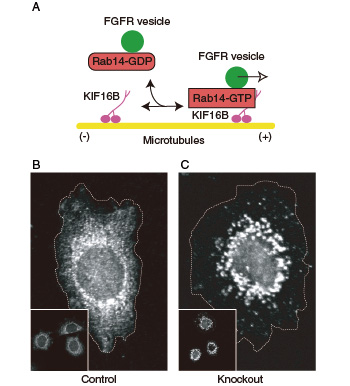
Fig.1 Transport of FGF receptor by the KIF16B molecular motor. (A) Rab14 adaptor on FGF-receptor-carrying vesicles is activated by GTP binding and become associated with the KIF16B molecular motor. This mechanism is essential for transporting FGF-receptor-carrying vesicles toward the plus ends of microtubule tracks at the cell periphery. (B and C) Intracellular distribution of FGF receptor. Compared with the control cells (B), KIF16B-knockout cells (C) showed perinuclear clustering of FGF receptor due to impairment of the anterograde transport.
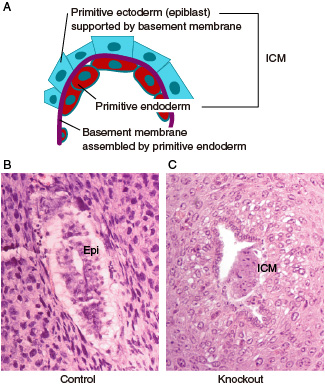
Fig.2 Development and differentiation of inner cell mass (ICM) due to the function of the KIF16B molecular motor. (A) FGF signaling facilitates differentiation of primitive endoderm from the ICM to secrete a basement membrane, which further supports the development of epiblast also derived from the ICM. (B and C) ICM differentiation in histological sections of implanted embryos on 5.5 days postcoitum. Although control embryos (B) developed egg cylinders containing epithelialized epiblast (Epi), primitive endoderm and epiblast of KIF16B-knockout embryos (C) could not be distinguished.
