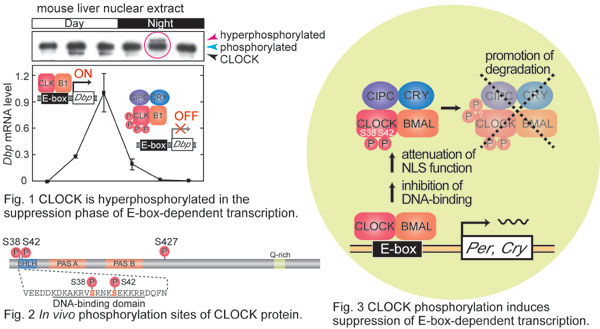
Roles of CLOCK phosphorylation in suppression of E-box-dependent transcription
In mammalian circadian clockwork, CLOCK-BMAL1 heterodimer activates E-box-dependent transcription, while its activity is suppressed by circadian binding with negative regulators such as CRYs.Yoshitane et al. in Fukada lab. found that CLOCK protein is hyperphosphorylated in the suppression phase of E-box-dependent transcription in the mouse liver (Fig. 1).CLOCK-BMAL1 purified from the mouse liver was subjected to MS/MS analysis, which identified Ser38, Ser42 and Ser427 as in vivo phosphorylation sites of CLOCK (Fig. 2).Ser38Asp and Ser42Asp mutations of CLOCK additively and markedly weakened the transactivation activity of CLOCK-BMAL1 with down-regulation of the nuclear amount of CLOCK and the DNA-binding activity.On the other hand, coexpression of CRY2 in NIH3T3 cells inhibited phosphorylation of CLOCK, whereas CIPC coexpression markedly stimulated the phosphorylation, indicating that CLOCK phosphorylation is regulated by a combination of the negative regulators in the suppression phase.CLOCKΔ19 lacking CIPC-binding domain was far less phosphorylated and much more stabilized than wild-type CLOCK in vivo.Calyculin A-treatment of cultured NIH3T3 cells promoted CLOCK phosphorylation and facilitated its proteasomal degradation.Together, CLOCK phosphorylation contributes to suppression of CLOCK-BMAL1-mediated transactivation through dual regulation; inhibition of CLOCK activity and promotion of its degradation (Fig. 3).
Program member
Yoshitaka Fukada (Department of Biophysics and Biochemistry, Graduate School of Science)