Temporal switching and cell-to-cell variability in Ca2+ release activity in mammalian cells
http://www.nature.com/msb/journal/v5/n1/full/msb20096.html
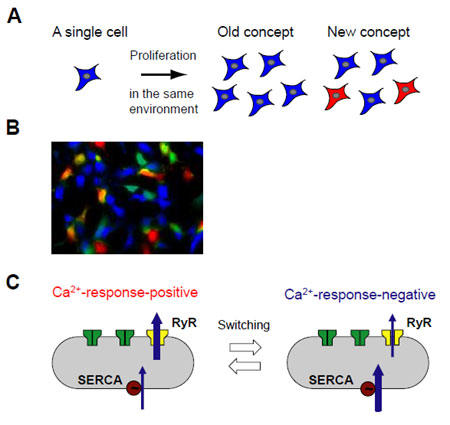
In recent years, cell-to-cell phenotypic variability within clonal populations (Panel A) has attracted considerable attention as a manifestation of gene expression noise, but that in differentiated mammalian cells remains largely unexplored. Here, we report that human embryonic kidney 293 cells exhibit all-or-none phenotypic variability in Ca2+ response upon agonist application: only approximately 40% of the cells respond to caffeine (Panel B). Using a systems-biological approach that combines time-lapse Ca2+ imaging with mathematical modeling, we showed that the positive feedback mechanism of “Ca2+-induced Ca2+ release” amplifies the small cell-to-cell differences in Ca2+ release and uptake activities and gives rise to all-or-none variability, and that individual cells switch between the positive-Ca2+-response state and the negative-Ca2+-response state with an average transition time of approximately 65 h (Panel C). A similar cell-to-cell variability was observed in smooth muscle cells in the portal vein of guinea pigs, suggesting a possible physiological role of the phenotypic variability in regulating portal vein contraction. Given the ubiquity of positive feedback loops and the versatility of Ca2+ signaling in mammalian cell signaling systems, it is possible that similar amplification mechanisms may contribute to cell-to-cell phenotypic variability in many cellular functions.
Program member
Masamitsu Iino (Department of Cellular and Molecular Pharmacology, Graduate School of Medicine)