PX-RICS, a chauffeur of cadherins -A novel mechanism of protein transport from the ER to the Golgi-
Cadherins mediate Ca2+-dependent cell-cell adhesion. Efficient export of cadherins from the endoplasmic reticulum (ER) is known to require complex formation with β-catenin. However, the molecular mechanisms underlying this requirement remain elusive. Here we show that PX-RICS, a β-catenin-interacting GTPase-activating protein (GAP) for Cdc42, mediates ER-to-Golgi transport of the N-cadherin/β-catenin complex. Knockdown of PX-RICS expression induced the accumulation of the N-cadherin/β-catenin complex in the ER and ER exit site, resulting in a decrease in cell-cell adhesion. PX-RICS-mediated ER-to-Golgi transport was dependent on its interaction with β-catenin, phosphatidylinositol-4-phosphate (PI4P), Cdc42 and its novel binding partner γ-aminobutyric acid type A receptor-associated protein (GABARAP). These results suggest that PX-RICS ensures the efficient entry of the N-cadherin/β-catenin complex into the secretory pathway, and thereby regulates the amount of N-cadherin available for cell adhesion.
Program member
estsu Akiyama (Institute of Molecular and Cellular Biosciences)
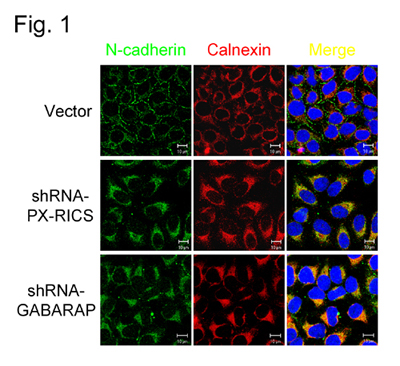
(Fig. 1) Knockdown of PX-RICS or GABARAP results in the disappearance of N-cadherin at the cell-cell boundaries and intracellular accumulation in the ER.
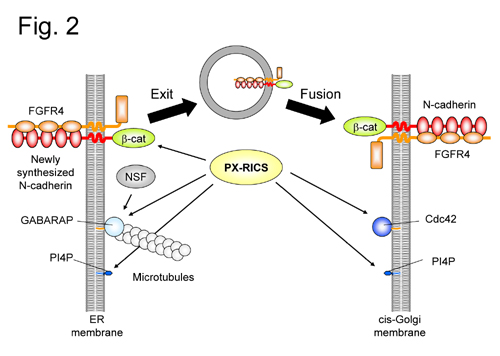
(Fig. 2) A hypothetical model for PX-RICS-mediated ER-to-Golgi transport of the N-cadherin/β-catenin/FGFR4 complex.
