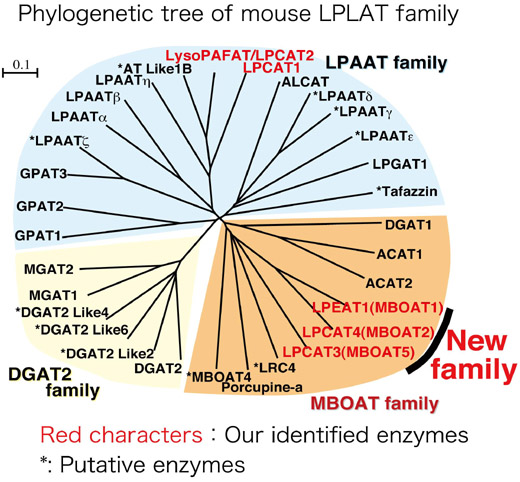
Discovery of a novel lysophospholipid acyltransferase family essential for membrane asymmetry and diversity
All organisms consist of cells, which are enclosed by cell membrane containing phospholipids. At present, membrane phospholipids are important not only as structural and functional components of cellular membrane, but also as precursors of various lipid mediators. Polyunsaturated fatty acids (PUFAs) comprising arachidonic acid or eicosapentaenoic acid are located at sn-2, but not at sn-1 position of glycerophospholipids in an asymmetrical manner. Additionally, the membrane diversity is also important for membrane fluidity and curvature. Rapid turnover of the sn-2 acyl moiety of phospholipids was described by Lands in 1958. However, the molecular mechanisms and biological significance of this remodeling pathway have remained elusive. Here, we identified a putative enzyme superfamily consisting mainly of three different gene families, which catalyze phospholipid biosynthesis from lysophospholipids and acyl-CoAs, acyl-CoA:lysophospholipid acyltransferases. Among new family (MBOAT family), we characterized three novel enzymes. According to tissue distribution and biochemical properties of these enzymes, they may collectively contribute to produce the different fatty acid composition of glycerophospholipids in cell membrane. Thus, our findings provide a novel and critical milestone in understanding how membrane diversity and membrane asymmetry are established and their biological implications for evolutionary changes.
Program members
Takao Shimizu (Department of Biochemistry and Molecular Biology, Graduate School of Medicine)
From Proc. Natl. Acad. Sci. USA, 2008
