Molecular mechanism how dendrite specific motor KIF17 releases NMDA receptors in an activity-dependent manner
Hirokawa's group previously revealed that KIF17, a kinesin superfamily protein, transports NMDA receptors through binding with Mint-1. It is well known that the amount of NMDA receptors on post-synaptic membrane increases in an activity-dependent manner. In this context, how KIF17 release NMDA receptors is a critical process that remains to be elucidated. Hirokawa's group reported that the tail region of the molecular motor KIF17 is regulated by phosphorylation. Using direct visualization of protein-protein interaction by FRET and various in vitro and in vivo approaches, it has been demonstrated that CaMKII-dependent phosphorylation of KIF17 on Ser 1029 disrupts the KIF17-Mint1 association and results in the release of the transported cargo from its microtubule-based transport.
Program member
Nobutaka Hirokawa (Department of Cell Biology and Anatomy, Graduate School of Medicine)
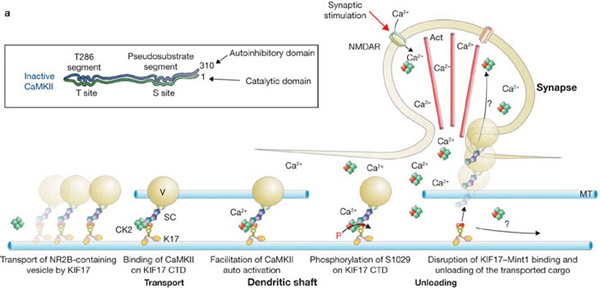
CaMKII, that is activated by stimulation, phosphorylates KIF17. Phosphorylated KIF17 dissociates from Mint-1.
