Phosphorylation of mammalian Sgo2 by Aurora B recruits PP2A and MCAK to centromeres.
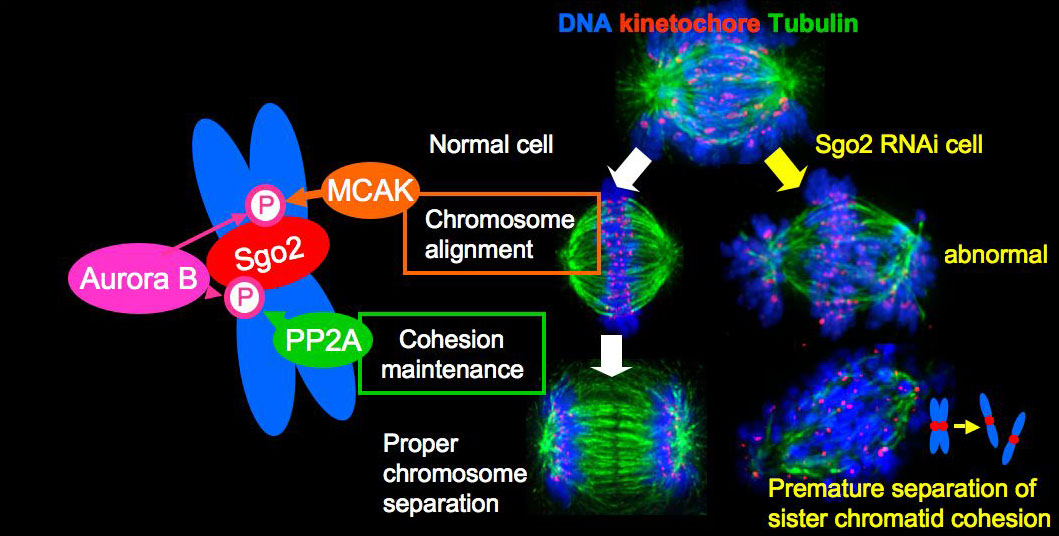
Shugoshin (Sgo) is a conserved centromeric protein. Mammalian Sgo1 collaborates with PP2A to protect mitotic cohesin from the prophase dissociation pathway. Although another shugoshin-like protein, Sgo2, is required for the centromeric protection of cohesion in germ cells, its precise molecular function remains largely elusive. We demonstrate that hSgo2 plays a dual role in chromosome congression and centromeric protection of cohesion in HeLa cells, while the latter function is exposed only in perturbed mitosis. These functions partly overlap with those of Aurora B, a kinase setting faithful chromosome segregation. Accordingly, we identified the phosphorylation of hSgo2 by Aurora B at the N-terminus coiled coil region and the middle region, and show that these phosphorylations separately promote binding of hSgo2 to PP2A and MCAK, factors required for centromeric protection and chromosome congression, respectively (Figure). Furthermore, these phosphorylations are essential for localizing PP2A and MCAK to centromeres. This mechanism seems applicable to germ cells as well. Thus, our study identifies Sgo2 as a hitherto unknown crucial cellular substrate of Aurora B in mammalian cells.
Program member
Yoshinori Watanabe (Institute of Molecular and Cellular Biosciences)
Figure
