Aberrant E2F Activation by Polyglutamine Expansion of Androgen Receptor in SBMA Neurotoxicity
Spinal and bulbar muscular atrophy (SBMA) is a neurodegenerative disorder caused by a polyglutamine repeat (polyQ) expansion within the human androgen receptor (AR). Unlike other neurodegenerative diseases caused by abnormal polyQ expansion, the onset of SBMA depends on androgen binding to mutant human polyQ-AR proteins. This is also observed in Drosophila eyes ectopically expressing the polyQ-AR mutants. We have genetically screened mediators of androgen-induced neurodegeneration caused by polyQ-AR mutants in Drosophila eyes. We identified Rbf (Retinoblastoma-family protein), the Drosophila homologue of human Rb (Retinoblastoma protein), as a neuroprotective factor. Androgen-dependent association of Rbf or Rb with AR was remarkably potentiated by aberrant polyQ expansion. Such potentiated Rb association appeared to attenuate recruitment of histone deacetyltransferase 1 (HDAC1), a corepressor of E2F function. Either overexpression of Rbf or E2F deficiency in fly eyes reduced the neurotoxicity of the polyQ-AR mutants. Induction of E2F function by polyQ-AR-bound androgen was suppressed by Rb in human neuroblastoma cells. We conclude that abnormal expansion of polyQ may potentiate innate androgen-dependent association of AR with Rb. This appears to lead to androgen-dependent onset of SBMA through aberrant E2F transactivation caused by suppressed histone deacetylation.
Program member
Shigeaki Kato (Institute of Molecular and Cellular Biosciences)
Tetsuya Tabata (Institute of Molecular and Cellular Biosciences)
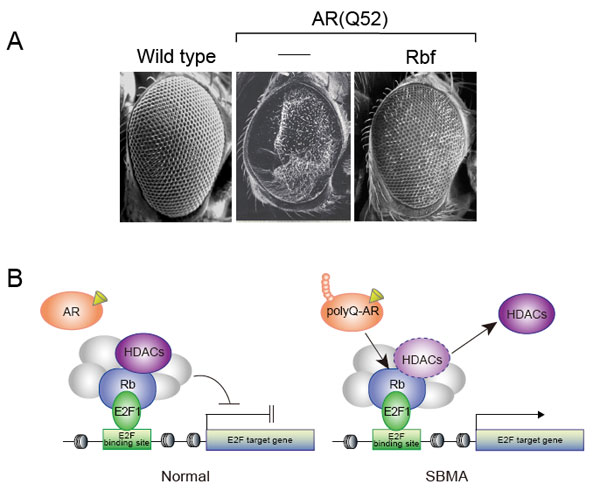
Aoverexpression of Rbf mutant lines rescue polyQ-AR-dependent neurodegeneration.
BAberrant E2F1 activation by a polyQ-AR mutant. Under normal conditions, Rb represses E2F1-mediated transcription through formation of an Rb/HDACs complex. In the presence of polyQ-AR, HDACs’ recruitment to Rb may be impaired, leading to aberrant E2F1 activation.
