Scaffolding function of PAK in the PDK1-Akt pathway
Akt is a key regulator of biological responses such as cell migration, cell growth, cell survival, metabolism and tumorigenesis, and its activity is tightly regulated. Although efficiency, localization and specificity of many signaling pathways are controlled by scaffold proteins, it remains unknown if such a mechanism is involved in regulation of the Akt pathway. Here we show that PAK serves as a scaffold protein in this pathway. PAK1 associates with both Akt and its T308 kinase PDK1 in response to growth factor stimulation. Expression of PAK1 induces T308 and S473 phosphorylation of Akt in a kinase activity independent manner. Upon growth factor stimulation, PAK1 and PAK2 are necessary for the efficient membrane recruitment and phosphorylation of Akt at both T308 and S473 as well as for the efficient association of Akt with PDK1. We also found that expression of DN-PAK1 reduced phosphorylation of only a subset of Akt substrates, detected by phospho-specific antibody to the Akt consensus sequence, and effectively reduced phosphorylation of FOXO3a, but not GSK3 or Bad, among the well-established Akt substrates. These findings reveal scaffolding functions of PAK that regulate the efficiency, localization and specificity of the PDK1-Akt pathway.
Program member
Yukiko Gotoh (Institute of Molecular and Cellular Biosciences)
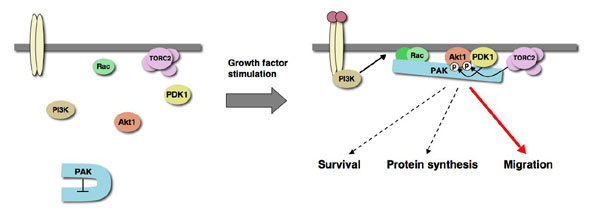
A working model for the role of scaffold protein PAK in regulation of the PDK1-Akt pathway
