Back to Lab members page
Japanese version, here
TERASHIMA Ichiro's studies
I have been studying on several subjects. Here, I summarize these studies briefly referring my papers. Many projects are going on in this laboratory.
(1) Light environment within a leaf and its effects on leaf photosynthesis. (1982 - present)
I examine optical properties of palisade tissue and spongy tissue isolated by means of paradermal sectioning from Camellia japonica leaves. Extinction coefficinet of chlorophyll in situ is greater in the spongy tissue than in palisade tissue (1-1). To have optically different two tissues in one leaf, the leaf can moderate the gradient of light absorption. However, the actual light gradient is considerable and chloroplats acclimate to their local light environments(1-2,-3,-4,and -5, Figs 1, 2).
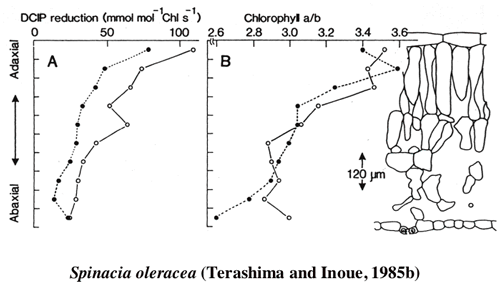 |
| Figure 1 Chlorophyll a/b ratio and the rate of electron transport rate per chlorophyll in 10 serially obtained sections from a spinach leaf. |
|
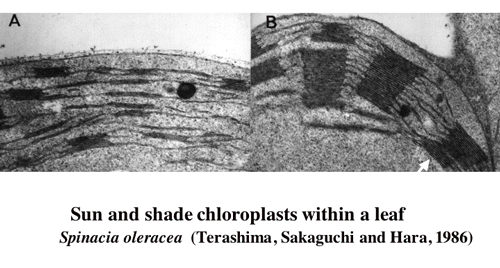 |
| Figure 2 Palisade tissue (left) and spongy tissue chloroplasts from a spinach leaf. |
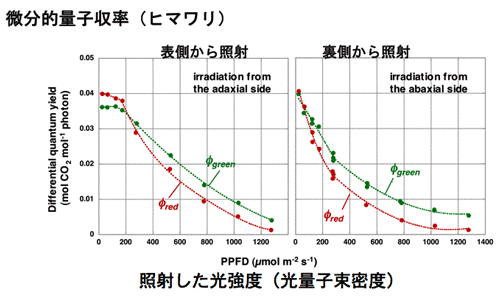
Effects of mesophyll differentiation into palisade and spongy tissue and chloroplast acclimation on the light response curve of leaf photosynthesis was modelled and the difference in curves depending on the illumination direction was discussed (1-6 and 1-7, Fig. 3).
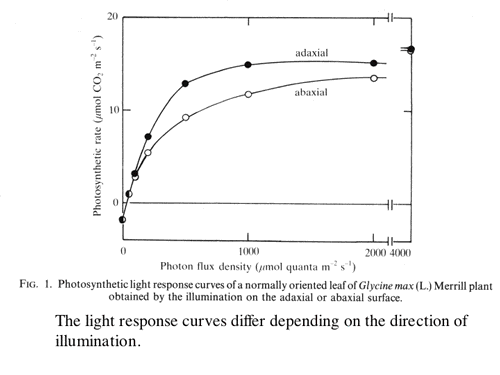 |
| Figure 3 Photosynthetic light response curves of a normally oriented leaf of Glycine max obtained by illuminating either adaxial or abaxial surface. |
Original Papers
- 1-1) Terashima, I. and Saeki, T. (1983) Light environment within a leaf I. Optical properties of paradermal sections of Camellia leaves with special reference to differences in the optical properties of palisade and spongy tissues. Plant and Cell Physiolgogy 24: 1493-1501.
- 1-2) Terashima, I. and Inoue, Y. (1984) Comparative photosynthetic properties of palisade tissue chloroplasts and spongy tissue chloroplasts of Camellia japonica L.: Functional adjustment of the photosynthetic apparatus to light environment within a leaf. Plant and Cell Physiology 25: 555-563.
- 1-3) Terashima, I. and Y. Inoue. (1985) Palisade tissue chloroplasts and spongy tissue chloroplasts in spinach: Biochemical and ultrastructural differences. Plant and Cell Physiology 26: 63-75.
- 1-4) Terashima, I. and Inoue, Y. (1985) Vertical gradient in photosynthetic properties of spinach chloroplasts dependent on intra-leaf light environment. Plant and Cell Physiology 26: 781-785.
- 1-5) Terashima, I., Sakaguchi, S. and Hara, N. (1985) Intra-leaf and intracellular gradients in chloroplast ultrastructure of dorsiventral leaves illuminated form the adaxial or abaxial side during their development. Plant and Cell Physiology 27: 1023-1031.
- 1-6) Terashima, I. and Saeki, T. (1985) A new model for leaf photosynthesis incorporating the gradients of light environment and of photosynthetic properties of chloroplasts within a leaf. Annals of Botany 56: 489-499.
- 1-7) Terashima, I. (1986) Dorsiventrality of photosynthetic light response curve of a leaf. Journal of Experimental Botany 37: 399-405.
Reviews
- Terashima, I. (1989) Productive structure of a leaf。In Photosynthesis, Edited by Briggs, W.R., pp. 207-226., Alan R. Liss, New York.
- Terashima, I., and Hikosaka, K. (1995) Comparative ecophysiology / anatomy of leaf and canopy photosynthesis. Plant, Cell and Environment 18: 1111-1128.
- Terashima, I., Araya, T., Miyazawa, S.-I., Sone, K. and Yano, S. (2005) Construction and maintenance of the optimal photosynthetic systems of the leaf, herbaceous plant and tree: An eco-developmental treatise. Annals of Botany 95: 507-519.
(2) Nitrogen use in the leaf and plant individual (1988 - present)
Not only the absolute level of nitrgen but the allocation pattern of nitrogen among photosynthetic components is important. In the shade, nitrogen is to be preferentially used in light harvesting system, while in the sun,it is mostly used to raise the maximum photosynthetic rate. are to beEffects of nitrogen nutrition and growth light environment on leaf photosynthesis (2-1, 2-2).
Hikosaka developed a model predicting apitimal allocation of nitrogen among the photosynthetic componets (2-3). The relevance of the model has been shown with herbaceous plants (2-4) as well as with woody plants (2-5).
There is a PPFD gradient within the plant stand, and old leaves that developed and act as sun leaves are shaded by younger leaves. Nitorgenous compounds in such old leaves are re-allocated to young leaves. The sensecence of old leaves is important in efficient nitrogen use. To examine whether light environmetn or leaf age is regulating sensecenc, Hikosaka devised a system with a vine plant that can be grown horizontally to avoid self-shading. He clearly showed that when nutrient level is sufficient, light environment is the primary determinant of leaf properties (2-6).It shoule be stressed here that senescence is acclimation process. When the (Hikosaka, K. (1996) Planta 198: 144-150).
Dr. Ono has been studying the mechanisms how a leaf senses its photosynthetic status in a plant. The Demand for photosynthates of the leaf would be sensed by monitoring sugar concentration in the leaf. Thus, she is examining the possibility that sugar-sensing is playing a role (see Ono's page). Araya is currently tackling with this problem. In particular, he is interested in sink-source transition. Tazoe is investigating nitrogen and light effects on organization of the photosynthetic machinery of C4 leaves.
Original papers
- 2-1) Terashima, I. and Evans, J.R. (1988) Effects of light and nitrogen nutrition on the organization of the photosynthetic apparatus in spinach. Plant and Cell Physiology 29: 143-155.
- 2-2) Evans, J.R. and Terashima, I. (1988) Photosynthetic characteristics of spinach leaves grown with different nitrogen treatments. Plant and Cell Physiology 29: 157-165.
- 2-3) Hikosaka, K. and Terashima, I. (1995) A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant, Cell and Environment 18: 605-618.
- 2-4) Hikosaka, K. and Terashima, I. (1996) Nitrogen partitioning among photosynthetic components and its consequences in sun and shade plants. Functional Ecology 10: 334-353.
- 2-5) Hikosaka, K., Hanba, Y.T., Hirose, T. and Terashima, I. (1998) Photosynthetic nitrogen-use efficiency in leaves of woody and herbaceous species. Functional Ecology 12: 896-905.
- 2-6) Hikosaka, K., Terashima, I. and Katoh, S. (1993) Effects of leaf age, nitrogen nutrition and photon flux density on the distribution of nitrogen among leaves of a vine (Ipomoea tricolor Cav. ) grown horizontally to avoid mutual shading of leaves. Oecologia 97: 451-457.
- 2-7) Ono, K., Terashima, I. and Watanabe, A. (1996) Interaction between nitrogen deficit of a plant and nitrogen content in the old leaves. Plant and Cell Physiology 37: 1083-1089.
Reviews
- Terashima, I., and Hikosaka, K. (1995) Comparative ecophysiology / anatomy of leaf and canopy photosynthesis. Plant, Cell and Environment 18: 1111-1128.
- Ono, K., Nishi, Y., Watanabe, A. and Terashima, I. (2001) Possible Mechanisms of Adaptive Leaf Senescence. Plant Biology 3: 234-243
- Terashima, I., Araya, T., Miyazawa, S.-I., Sone, K. and Yano, S. (2005) Construction and maintenance of the optimal photosynthetic systems of the leaf, herbaceous plant and tree: An eco-developmental treatise. Annals of Botany 95: 507-519.
(3) Photoinhibition (1988 - present)
When I was a postdoctral fellow in the laboratory of Professor Osmond, I started photoinhibition business. At first, we tried to cause of low temperature photoinhibition of photosynthesis. Unexpectedly, PS II in cucumber leaves, atypical chilling-sensitive plant, was resistant. After chilling treatment of the leaves in the light, I isolated thylakoids and found that thylakoids became leaky to protons. In vitro and in vivo studies clearly indicated that CF1 of H+-ATPase detaches from the membrane by chilling treatment in thelight (3-1, 3-2, and 3-3. but see 3-4, Fig. 4).
 |
| Figure 4 Traces of fluorescence induction curves in cucumber leaves before and after the light-chilling treatment obtained with a PAM system. A, untreated control; B, just after the light-chilling treatment for 5 hours; C, after the light-chilling treatment for 5 hours and the rewarming in the dark for 30 min. Note that the NPQ was lost after the light-chilling treatment. But NPQ was clearly observed after the rewarming. The plastoquinone pool was reduced in B and C due to the damage to PSI. |
These studies also clarified that this damage is reversible. However, the inhibition of photosynthesis is irreversible. Therefore, the uncoupling of the thylakoids is not a direct cause of the irreversible inhibition of photosynthesis.
Examining the partial reactions of photosynthesis, we found that PSI is damaged by the chilling treatment in the light (3-5). Dr. Sonoike further characterized this damage biochemically and biophysically (3-6, 3-7). Uncoupling during the chilling treatment in the light completely suppress ATP synthesis. Then Calvin cycle does not work. Furthermore, dissipation of energy as heat does not occur. Thus, electrons preferentially reduce O2. Because activities of scavenging enzymes are suppressed due to low temperature, H2O2 is not perfectly scavenged and by means of Fenton-type reaction is converted to hydroxyl radical. The hydroxyl radical thus produced damages bound-type iron sulfur centers (3-8).
For reviews see Dr. Sonoike's home page.
Dr. Inoue is studying molecular organization of PSII with Drs. Kaz. Sato and Kashino at Himeji Institute of Technology.
Original Papers
- 3-1) Terashima, I., Huang, L.-K., and Osmond, C.B. (1989) Effects of leaf chilling on thylakoid functions, measured at room temperature, in Cucumis sativus L. and Oryza sativa L. Plant and Cell Phsysiology 30: 841-850.
- 3-2) Wise, R.R., Terashima, I. and Ort, D.R. (1990) The effect of chilling in the light on photosphosphorylation. Analysis of discrepancies between in vitro and in vivo results. Photosynthesis Research 25>: 137-139.
- 3-3) Terashima, I., Kashino, Y. and Katoh, S. (1991) Exposure of leaves of Cucumis sativus L. to low temperatures in the light causes uncoupling of thylakoids. I. Studies with isolated thylakoids. Plant and Cell Physiology 32: 1267-1274.
- 3-4) Terashima, I., Sonoike, K., Kawazu, T. and Katoh, S. (1991) Exposure of leaves of Cucumis sativus L. to low temperatures in the light causes uncoupling of thylakoids. II. Non-destructive measurements with intact leaves. Plant and Cell Physiology 32: 1275-1283.
- 3-5) Terashima, I., Funayama, S. and Sonoike, K. (1994) The site of photoinhibition in leaves of Cucumis sativus L. at low temperatures is photosystem I, not photosystem II. Planta 193: 300-306 (1994).
- 3-6) Sonoike, K.,and Terashima, I. (1994) Mechanism of photosystem-I photoinhibition in leaves of Cucumis sativus L. Planta 194: 287-293.
- 3-7) Sonoike, K., Terashima, I., Iwaki, M., and Itoh, S. (1995) Destruction of photosystem I iron-sulfur centres in leaves of Cucumis sativus L. by weak illumination at chilling temperatures. FEBS Letters 362: 235-238.
- 3-8) Terashima, I., Noguchi, K., Itoh-Nemoto, T., Park, Y.-M., Kubo, A. and Tanaka, K. (1998) The cause of PS I photoinhibition at low temperatures in leaves of Cucumis sativus L., a chilling sensitive plant. Physiologia Plantarum 103: 295-303.
(4) CO2 diffusion in leaf photosynthesis. (1988 - present)
I characterized patchy photosynthesis over the leaf area in the presence of ABA (Fig. 5). When analyses using A-Ci relationship are made, some caution is needed (4-1).
 |
|
| Figure 5 The starch-iodine test. A sunflower leaf was illuminated in the presence of ABA. |
Dr. Hanba constructed an on-line carbon discrimination/gas exchange system. Leaves of evergreen trees (4-2) and alpine plants (4-3), the resistance to CO2 diffusion in the leaf is considerable so that the CO2 concentration in chloroplasts is very low in such plants.
Because of this resistance, sun leaves showing high photosynthetic rate needs to have large internal area for gas exchange (see Dr. Hanba's page). This explains why sun leaves are thicker than shade leaves (4-4, Fig. 6). But, we have not known how. Yano is studying this problem (see his page). In Chenopodium album, the number of cell layer in the palisade tissue increases in response to light environment of the mature leaves but not to that of the developing leaf (4-5, 4-6).
 |
| Figure 6 When a thin leaf accommodates a given amount of Rubisco per unit leaf area, chloroplasts are fat and the CO2 concentration in chloroplasts is low. This is why sun leaves having much Rubisco per unit leaf area should be thick. |
Recently we have got evidence that water channels in plasma membrane are involved in CO2 transfer (4-7, 4-8).
Original Papers
- 4-1) Terashima, I., Wong, S-C., Osmond, C.B. and Farquhar, G.D. (1988) Characterisation of non-uniform photosynthesis induced by abscisic acid in leaves having different mesophyll anatomies. Plant and Cell Physiology 29: 385-394.
- 4-2) Hanba, Y. T., Miyazawa, S.-I. and Terashima, I. (1999) Influences of leaf thickness on internal resistance to CO2 diffusion and d13C in leaf dry matter. Functional Ecology 13:632-639.
- 4-3) Kogami, H., Hanba, Y.Y., Kibe, T., Terashima, I. and Masuzawa, T. (2001) CO2 transfer conductance, leaf structure and carbon isotope composition of Polygonum cuspidatum Sieb. et Zucc, from low and high altitudes. Plant Cell and Environment 24: 529-537.
- 4-4) Teashima, I., Miyazawa, S. and Hanba, Y.T. (2001) Why are sun leaves thicker than shade leaves ? Jourunal of Plant Research 114: 93-105.
- 4-5) Yano S. and Terashima, I. (2001) Separate localization of light signal perception for sun or shade type chloroplast and palisade tissue differentiation in Chenopodium album. Plant and Cell Physiology 42: 1303-1310.
- 4-6) Yano, S. and Terashima, I. (2004) Developmental processes of sun and shade leaves in Chenopodium album L. Plant Cell and Environment 27: 781-793.
- 4-7) Terashima, I. and Ono, K. (2002) Effects of HgCl2 on CO2 dependence of leaf photosynthesis: Evidence indicating involvement of aquaporins in CO2 diffusion across the plasma membrane. Plant and Cell Physiology 43: 70-78.
- 4-8) Hanba,Y.T., Shibasaka, M., Hayashi, Y., Hayakawa, T., Kasamo, K., Terashima, I. and Katsuhara, M. (2004) Overexpression of the barley aquaporin HvPIP2;1 increases internal CO2 conductance and CO2 assimilation in the leaves of transgenic rice plants. Plant and Cell Physiology 45: 521-529.
Reviews
- Terashima, I. Anatomy of non-uniform leaf photosynthesis (Minireview). Photosynthesis Research 31: 195-212 (1992).
- Terashima, I., Ishibashi, M., Ono, K. and Hikosaka, K. (1996) Three resistances to CO2 diffusion: leaf-surface water, intercellular spaces and mesophyll cells. In Photosynthesis: from Light to Biosphere, Vol. V. Edited by Mathis, P. pp. V 537-542. Kluwer, Dordrecht.
- Terashima, I., Araya, T., Miyazawa, S.-I., Sone, K. and Yano, S. (2005) Construction and maintenance of the optimal photosynthetic systems of the leaf, herbaceous plant and tree: An eco-developmental treatise. Annals of Botany 95: 507-519.
(5) Photosynthesis in alpine plants
We made field measurements of photosynthesis in the East Himalayas (5-1). Because both O2 and CO2 partial pressures decreases with the decrease in atmospheric pressure, decrease in photosynthesis with elevation is small (5-2).
Mesophyll cell walls of alpine plants are thicker. This impose large resistance to CO2 diffusison (5-3).
Original Papers
- 5-1) Terashima, I., Masuzawa, T. & Ohba, H. Photosynthetic characteristics of a giant alpine plant, Rheum nobile Hook. f. et Thoms. and of some other alpine species measured at 4300 m, in the Eastern Himalaya, Nepal. Oecologia 95: 194-201 (1993).
- 5-2) Terashima, I., Masuzawa, T., Ohba, H., and Yokoi, Y. (1995) Does low atmospheric pressure in the alpine environment suppress photosynthesis ? Ecology 76: 2663-2668.
- 5-3) Kogami, H., Hanba, Y.Y., Kibe, T., Terashima, I. and Masuzawa, T. (2001) CO2 transfer conductance, leaf structure and carbon isotope composition of Polygonum cuspidatum Sieb. et Zucc, from low and high altitudes. Plant Cell and Environment 24: 529-537.
(6) Architecture of photosynthetic systems of trees.
Determination processes of characteristics of branches and those of leaves in relation to light environment were studied. We are planning to start to study tree architecture extensively. Suzuki is analyzing cost and benefit of tree growth (see Suzuki's page). Sone is intensively revising Shinozaki's pipe model (see Sone's page).
Original Papers
- 6-1) Kimura, K., Ishida, A., Uemura, A., Matsumoto, Y. and Terashima, I. (1998) Effects of current-year and previous-year PPFDs on shoot gross morphology and leaf properties in Fagus japonica. Tree Physiology, 18: 459-466.
- 6-2) Uemura, A., Ishida, A., Nakano, T., Terashima, I, Tanabe, H. and Matsumoto, Y. (2000) Acclimation of leaf characteristics in response to differences in previous-year and current-year light intensities in winter-deciduous Fagus species. Tree Physiology 20: 945-951.
- 6-3) Sone, K., Noguchi, K. and Terashima, I. (2005) Dependency of branch diameter growth in young Acer trees on light intensity and shoot growth activity. Tree Physiology 25: 39-48.
To the top of this page
Back to Lab members page







