Laboratory of Evolutionary Cell Biology Welcome to Shingyoji-Lab
Our Research
Life’s molecular motors - pursuing the motile mechanism of spermatozoa
Active movement is a sign of life in all plants and animals. It is found at all levels of their organization, that is, from the cells and tissues that make up their bodies to the whole organism. Although the movements of the organisms and their parts differ vastly in speed, force and other properties, most of them are driven by a few kinds of what are called “motor proteins” of the cell. There are three major kinds of motor proteins: myosin, kinesin and dynein. Among these, dynein is responsible for the movement of sperm cells, or spermatozoa, which propel themselves by moving their tails or flagella. In flagella as well as in other parts of the cell, the function of dynein is to move along a microtubule, one of the cytoskeletal components of the cell, by using energy obtained through the hydrolysis of ATP.
Sperm cells swim towards the egg by “beating” their flagella. In sea urchin sperm, for example, rhythmic bending waves are formed at the base of the flagellum at about 40-50 cycles/sec and propagated towards the tip. Sperm can change their direction and swimming speed by changing their waveform.
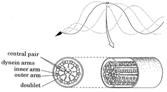
The internal structure of the flagella responsible for the movement is the axoneme. It consists of microtubules and other components arranged in the so-called “9+2” pattern, in which nine ‘doublet’ microtubules surround the ‘central-pair’ microtubules. Dynein arms occur in two regular rows along each of the nine doublet microtubules. They cause sliding movement of adjoining doublet microtubules past one another. The flagellar bending waves are produced by regulated activity of the dynein arms.
What causes the flagella to oscillate? This is one of the questions that have not been answered for a long time. Recently, we measured the force generated by dynein with ‘optical trap nanometry’, and found that a single dynein arm in its natural position on the doublet microtubule generated a force of about 6 pN. More significantly, the force developed by a single dynein molecule showed oscillation (Nature, 393: 711-714, 1998). The oscillatory properties of individual dynein molecules may be important for flagellar oscillation as well as the control mechanism in which the higher level ‘9+2’ structure of the axoneme are generally implicated.
How does the ‘9+2’ structure regulate microtubule sliding to produce oscillatory flagellar bending? Our recent study with elastase-treated sea urchin sperm flagella (J. Cell Sci., 116: 1627-1636, 2003) has shown that the dynein arms on one side of the axoneme and those on the opposite side are alternately activated to induce the reciprocal sliding necessary for oscillatory bending. The switching of the activity of dynein is regulated by the central-pair of microtubules. This is the basis for the regulation of both the direction and the speed of swimming sperm.
 |
 |
 |
| 1-a | 1-b | 1-c |
Fig. 1
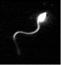
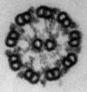
(a) A sea urchin sperm; (b) electron micrograph of a cross-section of its flagellum (the membrane has been removed to expose the axoneme); (c) schematic drawing of the flagellar axoneme showing the 9+2 structure within membrane.
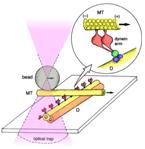 |
Fig. 2
Drawing showing the principle of optical-trap nanometry used to measure the force of a single dynein arm.
Regulation of dynein activity in flagellar motility
Dynein is not a simple motor converting the chemical energy of ATP hydrolysis into the mechanical work of microtubule sliding but has a built-in regulatory mechanism. In sea urchin sperm flagella, microtubule sliding necessary for the oscillatory bending is the result of alternate activation of dynein between the two sides of the central pair (CP). This pattern of activation is reflected in the characteristic sliding disintegration of elastase-treated flagellar axonemes into two doublet bundles of unequal thickness. This, however, occurs only under high (‘physiological’) ATP conditions. Regardless of the presence of ATP, the motile activity of dynein arms is low except for those of the doublet microtubules on the two sides of the CP. These dynein arms are, however, activated if ADP is present in addition to ATP.
The motor activity of dynein can also be modified by imposed bending of the doublets. Our previous study identified the mechanical effect of bending as a key step in the self-regulatory mechanism controlling microtubule sliding so as to generate flagellar oscillation (Current Biol., 14: 2113-2118, 2004) (Fig. 3). Recent study has demonstrated that mechanical bending also triggers active bending in demembranated motionless flagella even at very low ATP concentrations (1.5-3 μM). For the induction of continuous beating, however, the presence of ADP is necessary (Cell Struct. Funct., 32: 17-27, 2007). Thus, the CP and the mechanical force as well as nucleotides (ATP and ADP) are important factors in the regulation of the activity of dynein to produce flagellar oscillation.
By using a fluorescent ATP analogue, we found that both noncatalytic ATP binding and stable ADP binding, in addition to ATP hydrolysis, are involved in the regulation of the chemo-mechanical transduction in axonemal dynein (Cell Motil. Cytoskel., 64: 690-704, 2007) (Fig. 4). Our recent study on the roles of ATP and ADP in the regulation of normal beating in demembranated flagella has shown that the regulation of on/off switching of dynein motor activity involves ATP-inhibition and ADP-activation, probably through protein phosphorylation and dephosphorylation (Cell Motil. Cytoskel., 64: 777-793, 2007). This mechanism may also form the basis for the regulation of dynein activity by mechanical force and by the CP.
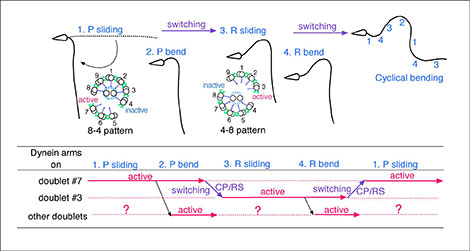 |
Fig. 3
A model for the self-regulatory feedback mechanism for flagellar oscillation.
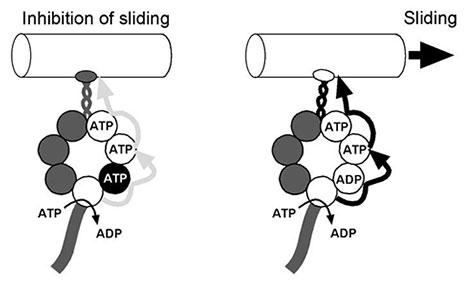 |
Fig. 4
A model for dynein-nucleotide interactions. ATP-dependent inhibition (left). Active state (right) induced by noncatalytic ATP binding and stable ADP binding.
Remaining questions
Recent studies made in many laboratories, including ours, have elucidated much of the basic mechanisms underlying sperm motility. There are, however, still many unsolved problems regarding the regulation of dynein in flagellar motility. One of the remaining questions is how the oscillation of dynein is related to the flagellar oscillation. Further studies of the dynamic properties of both dynein and the doublet microtubules will give us a clue to solving these problems.